Radiological Imaging
Diagnostic Radiological Imaging Contribution To Translational Medicine
The last 10 years have seen the emergence of a major conceptual change spanning across several medical disciplines. This movement has led to the creation of translational medicine.
The concept of translational medicine is based on an effort to bridge preclinical and clinical trial phases for strictly similar biological targets (and consequently clinically pertinent) and on monitoring therapeutic treatments, both for developing new molecular entities and monitoring responses to treatment through biomolecules already on the market. Translational research constitutes a very positive trend for medical imaging, and in particular, molecular imaging.
Molecular imaging makes it possible to visualize processes that are the very basis of life. The revolution within molecular biology has contributed in recent years to the development of imaging, making it possible to understand the inner mechanisms of cell function within its environment. The ability to be able to perform this identification within a living organism is the objective of molecular imaging.
Major Trends In Molecular Imaging
“Functional” imaging is currently in a phase of significant expansion in response to a need to describe with precision certain biological processes, within the framework of anti-tumour products;eg.antiangiogenic or antivascular molecules. The benefits of this approach are substantial, contributing significantly to a better understanding and diagnosing pathological processes. Furthermore, it leads to the development of new treatments as well as monitoring their effect on patients.
It is easy to anticipate that these two concepts (that are of course linked) will in the medium term considerably contribute to advances in medical imaging:
- Medicine referred to as “personalized” requiring the availability of validated biomarkers;
- “Theranostics” is the association of a targeted therapeutic agent and an agent with a targeted diagnostic application, or an agent contributing to monitoring the efficacy of treatment.
- Anatomical Imaging
- Functional Imaging
- Molecular Imaging
I. Cardiovascular Diseases Key Facts
Cardiovascular illness is the leading cause of mortality in the world (29% of mortality worldwide) or 17.1 million deaths[1]a
- 42% of these deaths are the result of coronaly heart disease
- 33% of these deaths are the result of a cerebral vascular accident (CVAs)[2]
Needs
Preventing the risk of CVAs
The Trial Challenges
Develop a clinical trial product capable of discriminating at risk from stable plaques. The rupture vulnerable plaque is one of the primary causes of CVAs.
*Sources:
(1) World Health Organization (WHO), September 2009.
(2) World Health Organization (WHO), most recent statistics, 2004
II. Cancer Key Facts
2nd cause of death in the European Union (1)
3.4 million new cases of cancer diagnosed and 1.8 million deaths caused by cancer in Europe in 2008.(2)
Needs
Adapting treatments to the patient (personalized medicine). Identifying the incidence of recurrence reliably.
The Trial Challenges
Developing a specific product for the detection of ovarian cancer recurrence and the monitoring treatments.
Developing an agent to evaluate prognosis before treatment and monitor the antitumor therapies more specifically targeted therapies.
*Sources:
(1)International Agency for Research on Cancer (IARC), Lyon, 2007
(2)International Agency for Research on Cancer (IARC), Lyon, 2008. Word Cancer Report 2008
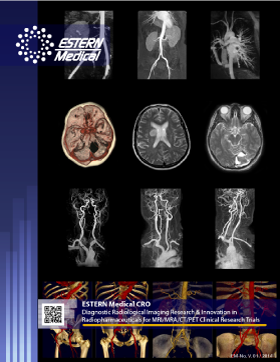
Examples of key molecular imaging applications
III. INFLAMMATORY AND NEURO-DEGENERATIVE DISEASES KEY FACTS
In the USA & Europe, Alzheimer’s disease affects more than 14 million people.
The total cost of healthcare for patients affected by this disease is estimated at $100 billion US/Dlls per year.
Needs
Contribute to early and reliable diagnosis of Alzheimer’s disease to improve treatment responses.
Facilitate evaluation of the efficacy of new treatments.
The Trial challenge
Develop a contrast agent to detect neural inflammatory processes associated with Alzheimer’s disease lesions.
Developing a specific product allowing direct visualization of lesions and contributing to improve monitoring of treatment.
A major research initiative
- Our goal is to push the boundaries of MRI applications
- Contribute to the evolution of MRI to meet new diagnostic requirements
- Move from anatomical to molecular imaging
- Increase sensitivity in contrast and spatial resolution
- Meet the challenges posed by increased magnetic fields
- Adopt an integrated vision of the technology product machine concept
Conclusion
Since the early 2000’s, ESTERN Medical CRO has contributed to major advances in clinical trials medical Diagnostic Radiological Imaging to achieve improved patients care throughout the world.
Through its recognized expertise in CT, PET and MRI and its extensive product range (MRI and Nuclear Medicine), ESTERN Medical assists in the Pharmaceutical, Biotech & Medical Device R&D of healthcare companies and professionals (radiologists, cardiologist, oncologists…) in achieving better diagnosis of patients and better targeting of their treatment.
ESTERN Medical intends to deploy its scientific and clinical expertise and innovation in the service of the major Pharmaceutical and Medical Device health care challenges of the 21st century. Our mission is to guide, support and contribute as a CRO to advances in diagnosing major pathologies by proposing innovative and effective imaging trial solutions to achieve improved patient care throughout the world.
We know that future products will target anomalies at the molecular level and will make it possible to apply personalized treatment solutions for highly disabling pathologies. To achieve this objective and invent a new generation of contrast agents, ESTERN Medical Research and Clinical Development teams, to maximize their own efforts at innovation, are supported by many years of productive scientific and technological partnerships, notably through highly reputed international Research Networks of Excellence.
This commitment to innovation focused on improving the care and treatment provided to patients also requires close collaboration with sponsors and clinical practitioners.
Our Knowledge is in:
- Evaluating cardiovascular risk
- Achieving more precise diagnosis of tumors and evaluating the therapeutic response
- Early detection of Alzheimer’s disease
Our Broad Radiological Trial Experience
ESTERN Medical personnel and senior executive management provide Pharmaceutical & Medical Device companies with considerable experience using radiological imaging as part of clinical trials.
In Latin America we have successfully managed a wide range of radiological imaging studies across multiple imaging modalities looking at a diverse a number of indications; products that are now on the global market.
Our Net Radiological Imaging Coverage Across Latin America
ESTERN Medical is able to offer scientific and senior team members that not only have considerable hands-on clinical trial experience, but who also have excellent relationships
with imaging investigators and key opinion leaders.
With rapid access to eligible patients across a wide geographical area – ESTERN Medical has the unique ability to tap into underutilized patient populations in diverse locations in Latin America – which has allowed us to build a reputation as the primary CRO to be relied upon to meet or exceed patient recruitment targets.
This includes considerable experience in securing patients for studies where timely recruitment has been a problem, through opening up sites in new geographies and working with clinical sites that have a track record for rapid recruitment.
We utilize our proprietary database of key clinical sites to drive recruitment targeting specific or general patient populations for any indication.
Our Cro Advantage
With a heritage specializing in imaging, we have Successfully managed more imaging compounds than any other CRO across Latin America for Global Sponsors.
Our management team are respected professionals in clinical imaging, regulatory affairs, radiology technology, clinical operations, business development, and clinical trial management.
Our local Monitors and Regulatory Affairs Associates in each of the regional countries will expedite study start-up and facilitate excellent relationships with the sites.
Our years of experience working in the emerging market countries across Latin America and our excellent long-term relationships with the local regulators allows us to offer rapid study start-up and provide access to qualified sites with which we have successfully worked with on numerous occasions. This enables us to provide our sponsors with rapid patient recruitment and quality data to assure a high standard of study conduct.
Our Strong Project Management ensures that studies are run efficiently, maintaining consistency across sites and providing quality deliverables. We offer highly experienced professionals with many years of experience to manage these studies, and to develop contingency plans to assure timely completion of these studies within budget and to the highest quality standards